a Institute of Chemical Physics RAS
Kosygin st. 4, 117977 Moscow B-334, Russia
b Institute of General & Inorganic Chemistry, Belarusian Academy of Sciences, Minsk 220072
c M.Lomonosov Moscow State University, Department of Chemistry, Moscow 117234, Russia
Nanostructured oxidic semiconductors such as TiO2,
ZrO2, etc., were widely used as photoelectrochemical electrodes and colloidal
photocatalysts for decomposition of toxic pollutants [1]. The importance of the hole-electron
recombination kinetics in determining the photocatalytic activity of colloidal semiconductors has
been discussed [2,3]. To improve photochemical properties of the photocatalysts, doping of their
surface with transition metal ions was usually applied [1-3]. An EPR study of TiO2
aqueous colloids doped with Fe, V or Mo has been reported [4]. The authors observed inhibition
of hole-electron recombination by Fe3+ and V4+ dopants.
One of the most important characteristics of the doped semiconductor particles for better
understanding of their properties and action is knowledge about the structure and spatial
distribution of the dopant centers with measurement of the distances < r > between them
or their local concentration < C >. The EPR technique allows to obtain such data in the case
of paramagnetic centers (PC) in any diamagnetic matrix [5]. In our previous work [6] we have
presented some quantitative data for polycrystalline TiO2 electrodes doped with
V4+ and Cr3+ ions. The method for < C > and < r >
determination from magnitude of the dipole-dipole interaction in cases of random, cubic or pair
spatial distribution is well known and was proved experimentally in numerous works. The
problem of determination the parameters analogous to < C > and < r > for systems in
which PC were distributed on the surfaces of solid materials or in the interface is not solved yet.
In this paper we perform our recent results obtained for the nanosized TiO2
(Degussa P 25, Hombicat-100 and nano-TiO2 prepared ourselves) and
ZrO2 particles contained different amount of V(4+) ions on the surface. For the
correct calculation of the corresponding parameters < Csurf > and <
rsurf > a special theoretical and computer analysis has been done.
 |
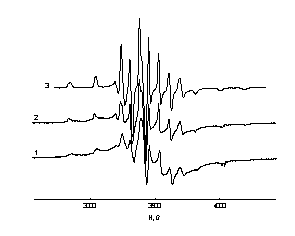
Fig.1. EPR spectra of the P-25 particles after: 1 – 10 min, 2 – 6 days,3 –75 days of incubation in 0.15 cm3 of 0.65 M ascorbic acid in C2H5OH-H2O = 3:2 solution. [V4+] = 1.8 x1020 cm–3; T = 77 K. |
Fig.1 presents typical EPR spectra of TiO2 (Degussa P25) particles at
different content of V4+ ions. It is evidently seen, that, as we have observed for the
doped matrix of polycrystalline TiO2 electrodes [6], there are two types of
V4+ dopants: a) 'isolated' centers with well resolved anisotropic EPR spectrum and
rather low concentration < Csurf > of them and b) 'aggregates' with a broad
singlet line in the EPR spectrum usual for PCs with strong spin-exchange interaction between
them and much shorter distances < rsurf > comparing with 'isolated' centers.
Similar results were obtained for all TiO2 and ZrO2 systems, but
appearance of the singlet line depended strongly on the vanadium content.
Figures 2 and 3 present changes in the low-field parts of the V4+ EPR spectra
for the samples prepared using Degussa P 25 and Hombicat-100 powders at various
V4+ contents (Fig. 2) or time after sample’s preparation (Fig. 3). The main
regularities of the structural features of V4+ centers in the TiO2 and
ZrO2 interface will be discussed in the paper.
 |
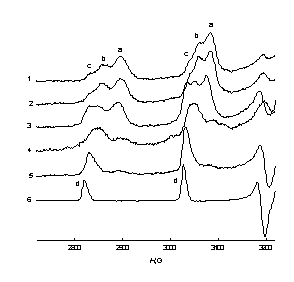 |
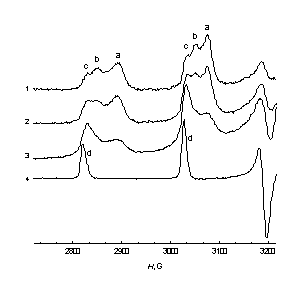
| Fig.2. Low-field lines of the EPR spectra of the Hombicat-100 particles with [V4+] content: 1 – 1.6 ×1019 cm–3, 2 – 4.0 ×1019 cm–3, 3 – 1.2 ×1020 cm-3 after 15 days of incubation in 0.15 cm3 of 0.75 M ascorbic acid; 4 –0.01M VO2+ in C2H5OH-H2O = 3:2 solution; T = 77 K. | Fig.3. Low-field lines of the EPR spectra of the Hombicat-100 ( 1-3 ) and P-25 ( 4,5 ) particles after: 1 – 10 min, 2, 4 – 25 h, 3, 5 –15 days incubation in 0.15 cm3 of 0.75 M ascorbic acid. [V4+] = 4.0 ×1019 cm–3 ( 1 – 3 ) and 2.0 ×1019 cm–3 ( 4 , 5 ); 6 –0.01M VO2+ in C2H5OH-H2O = 3:2 solution; T = 77 K. |
One can see from these figures that there exist up to three types of surface V4+ centers with slightly different spectra and spin-Hamiltonian parameters. Analysis of these changes showed that in time V4+ ions from “aggregates” are transformed mainly to the “c” type of the “isolated” centers (Fig. 2, 3), though these “c” ions are still anchored to the surface, and not dissolved in a liquid phase. An attempt to attribute species “a-c” has been done, as well as to characterize quantitatively the peculiarities of spatial distribution of the V4+ ions on the oxidic surfaces.
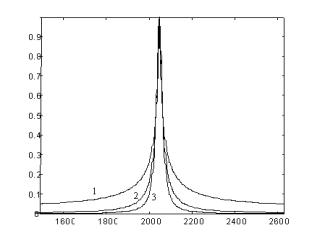 |
Fig. 4. Fourier transformed dipole frequency spectra for 1-, 2- and 3-dimentional distributions of PCs. |
For the correct calculation of corresponding parameters < C surf> and <
rsurf> for the studied systems, a special computer analysis has been carried out.
It was shown (Fig. 4) that there existing the essential difference in the EPR-line shape for three
cases of 3-, 2- and 1- dimensional systems. The obtained experimental results will be discussed
in the paper in detail and will be compared with structural data known for the TiO2
and ZrO2 crystalline materials doped with V4+ inside the bulk matrix.
It was theoretically shown that there existing the essential difference in determination of
the structural parameters for three-, two- and especially one-dimensional systems [7, 8]. The
following equation performs the relation between T 2 D and nD :
T2 D-1= 4.64 nD3 / Dg2ħ ,
where T2D is the transversal relaxation time of electron spin, g is an electron magnetogyric ratio, nD is D -dimensional density of paramagnetic centers and D is the dimension of the PC’s spatial localization [8].
Thus, comparing the structural properties of metal-doped oxidic
semiconductors one can conclude that:
1) a tendency to form the metal ion aggregates with high local concentration < Csurf>
of the doping ions is typical for V4+ species on the surface
of TiO2 and ZrO2 nanoparticles, similar to what has been observed
for a matrix of V4+-doped polycrystalline TiO2 systems studied in [6].
2) Relative concentrations of different V4+ species of the 'isolated' and
'aggregated' V4+ ions depend on vanadium content in the sample and the nature of the carrier.
Various types of V4+-doped TiO2 nanoparticles, as well as of ZrO2 ones, presented different peculiarities of the structure and spatial distribution of metal centres on their surface, that should be taken in consideration for explanation of photocatalytic and photoelectrochemical behaviour of such nanocrystalline systems.
Acknowledgements:
The authors are grateful to the RFBR (grant No.00-03-81168) and INTAS (Grant No.
00-0863) for financial support. We are thankful to Prof. A.Kh. Vorob’ev for providing us his
PC EPR-programs and to Prof. M.Ya. Melnikov for valuable discussions.
References
| [1] | Photoelectrochemistry, Photocatalysis and Photoreactors. M. Schiavello, Ed. Dordrecht: Reidel Publ. Co., 1985; Photochemical Conversion and Storage of Solar Energy. E. Pelizzetti, M. Schiavello, Eds. Dordrecht: Kluwer, 1991. |
| [2] | Kalyanasundaram K., Gratzel M., Pellhzetti E., Coord. Chem. Rev., 69, 57 (1985). |
| [3] | Martin S.T., Morrison C.L., Hoffmann M.R., J. Phys. Chem., 98, 13695 (1994). |
| [4] | Grätzel M., Howe R.F., J. Phys. Chem., 94, 2566 1990). |
| [5] | Abragam A., The principles of nuclear magnetism. Clarendon Press, Oxford, 1961. |
| [6] | Kokorin A.I., Arakelian V.M., Arutyunian V.M., Proc. of 13-th QUANTSOL, Kirchberg, Austria, 2001, p. 13-15. The same , Russian Chem. Bull., 2002, in press. |
| [7] | Dzeparov F.S., Lundin A.A., Khazanovich T.N., Russion JETP, 92, 342 (1987). |
| [8] | Atsarkin V.A., Vasneva G.A., Demidov V.V., et al., JETP Letters, 72, 530 (2000). |
email: kokorin@chph.ras.ru